
Purdue Pharma’s sales reps in Tennessee were pushing OxyContin even harder as the high-powered opioid came under more legal scrutiny in recent years. That’s among hundreds of claims made by the Tennessee Attorney General in a lawsuit filed in May and made public Thursday.
Purdue withdrew its effort to keep the litigation under seal, citing trade secrets. That prompted the Tennessee AG to
publish the suit online. The Tennessee Coalition for Open Government had also
intervened to advocate for opening the process.
“We are pleased Purdue Pharma decided to withdraw its motion for a protective order, and the State’s complaint is now available to the public,” Attorney General Herbert Slatery said in a statement. “We believe the public has the right to know what is going on in this important lawsuit.”
Purdue’s 87 sales reps in the state made 300,000 calls to Tennessee prescribers over the last decade, and the pace didn’t peak until 2016 — well after Purdue’s tactics were under investigation.
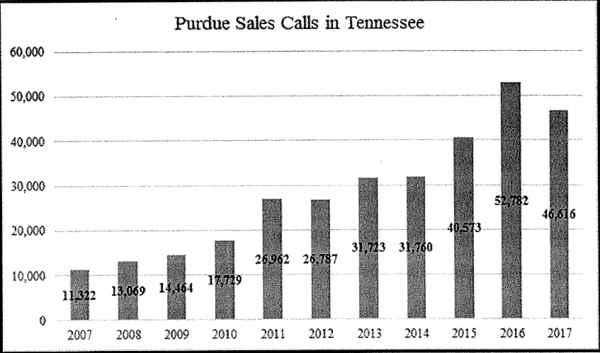
Tennessee’s lawsuit depends heavily on four-line blurbs sales reps jotted down after each visit or phone call. Some reveal suspicious activity, like patients paying cash and a knife fight outside a doctor’s office in Springfield. And in that specific instance, Purdue reps still called on the doctor 31 times after his medical license was suspended.
According to the lawsuit, Purdue reps also continued to call on doctors after:
- Law enforcement identified two particular doctors responsible for significant diversion
- Credible reports of patient overdoses
- A provider admitting to heroin addiction
- Muggings over controlled substances outside a pharmacy linked to a provider
- Admission by a provider he was running a pill mill
- Observing a patient being coached in a waiting room
- Choreographed pill counts and urine screenings
- Standing-room-only waiting rooms
Other revelations from the Tennessee evidence:
- Notes from a sales rep in training include the line, “Never give someone more info than they need to act.”
- “We sell hope in a bottle,” was how Purdue summarized the marketing for its opioid products in a hiring guide.
- Sales team increasingly focused on nurse practioners, physician assistants and family practitioners who lacked pain management expertise. They made up 65 percent of all prescribing in Tennessee.
- Allegedly, the biggest misleading claim by sales reps was that there was no limit to how much OxyContin they could prescribe a patient.
- In total over the decade, 104 million OxyContin tablets were prescribed in Tennessee and half of patients were taking more than the CDC recommended dose.
- At least five problem prescribers are named — Dr. Michael Rhodes, Dr. James Pogue, Dr. Mireille Lalanne, Dr. Visuvalingam Vilvarajah and Dr. Donald Boatright.
Nashville attorney Mark Chalos of Lieff Cabraser says the same evidence is being used by his firm to sue opioid makers on behalf of counties and cities.
“I think it helps all of us engaged in this fight to better understand what has happened and ultimately to get more quickly and more efficiently to a resolution,” Chalos says.
With so much litigation at varying stages, it’s unclear what suits will go to trial or be settled. But legal experts expect big money could change hands. Tennessee is asking for $10,000 for every time Purdue “knowingly” violated consumer protection law.
A spokesman for Purdue Pharma says in a written statement the company “vigorously” denies that its sales force acted improperly and that it’s disappointed Tennessee is pursuing a separate lawsuit outside of collective negotiations with other states.
“The Attorney General claims Purdue acted improperly by communicating with prescribers about scientific and medical information that FDA has expressly considered and continues to approve. We believe it is inappropriate for the state to substitute its judgment for the judgment of the regulatory, scientific and medical experts at FDA.”
Purdue
stopped all sales force activity related to opioids in February.


