A Nashville cancer surgeon wanted to be part of the COVID-19 clinical trial, and found out Wednesday he received the placebo. So when Pfizer “unblinded” the study and told health care workers they could get the real thing, Dr. William Polk was first in line.
Because Tennessee hospitals are getting their shipments of the vaccine later than expected, Polk is likely first person in the state to receive the vaccine outside a clinical trial. He says being the first makes him reflect on what he calls the “miraculous” timeline.
“To have, after less than a year, a vaccine that has really fantastic efficacy in preventing something that has completely upended our lives, yeah it matters,” he tells WPLN News.
Polk, who is 62, says he wasn’t surprised to learn he was part of the control group of Pfizer’s trial because he felt no side-effects, which tend to be more severe than with most vaccines.
Polk is one of 29 health care workers identified by Nashville-based Clinical Research Associates, which has run a trial site for Pfizer in Nashville with more than 300 patients. They recruited health care workers since they are more likely than many people to be exposed to COVID-19 and test the shot’s effectiveness.
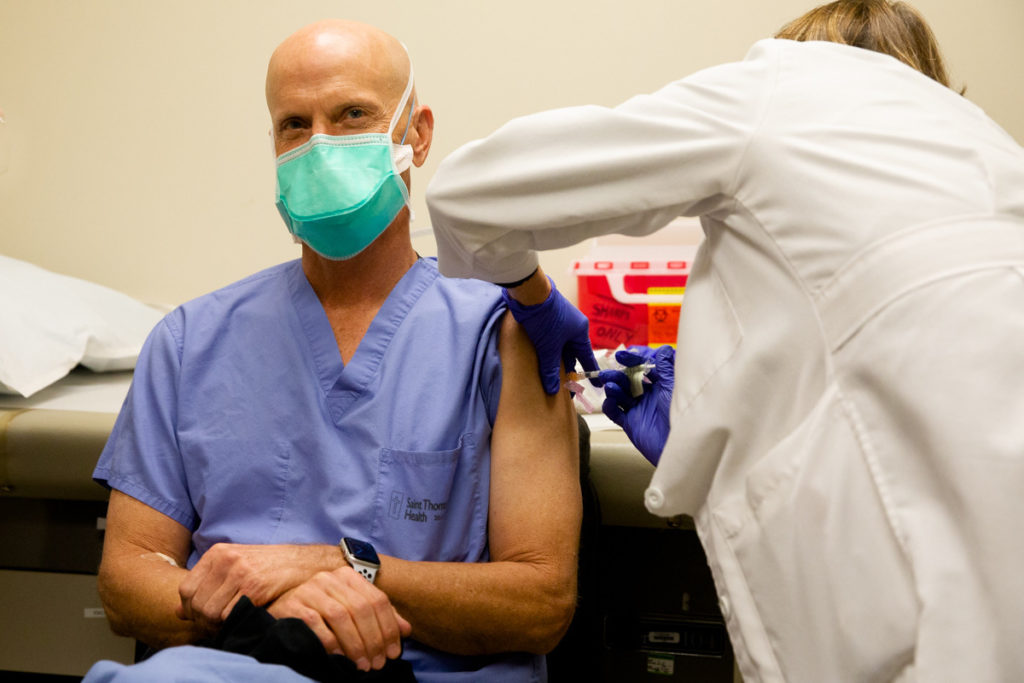
Polk returned to CRA on Church Street to get the real vaccine.
“Alrighty,” Polk said as a flurry of camera shutters clicked. “Didn’t feel a thing.”
Tennessee’s largest hospitals, including TriStar Centennial Medical Center and Saint Thomas Midtown where Polk works, are planning to start vaccinating front-line workers starting Thursday. But they had been told they would have their first doses earlier this week.
About 56,500 doses of the Pfizer vaccine are expected, including 5,000 at Vanderbilt University Medical Center. Tennessee is one of the last states to start administering the COVID vaccine on a large scale.
Another 115,000 vaccines from the drug company Moderna are expected to arrive next week.

